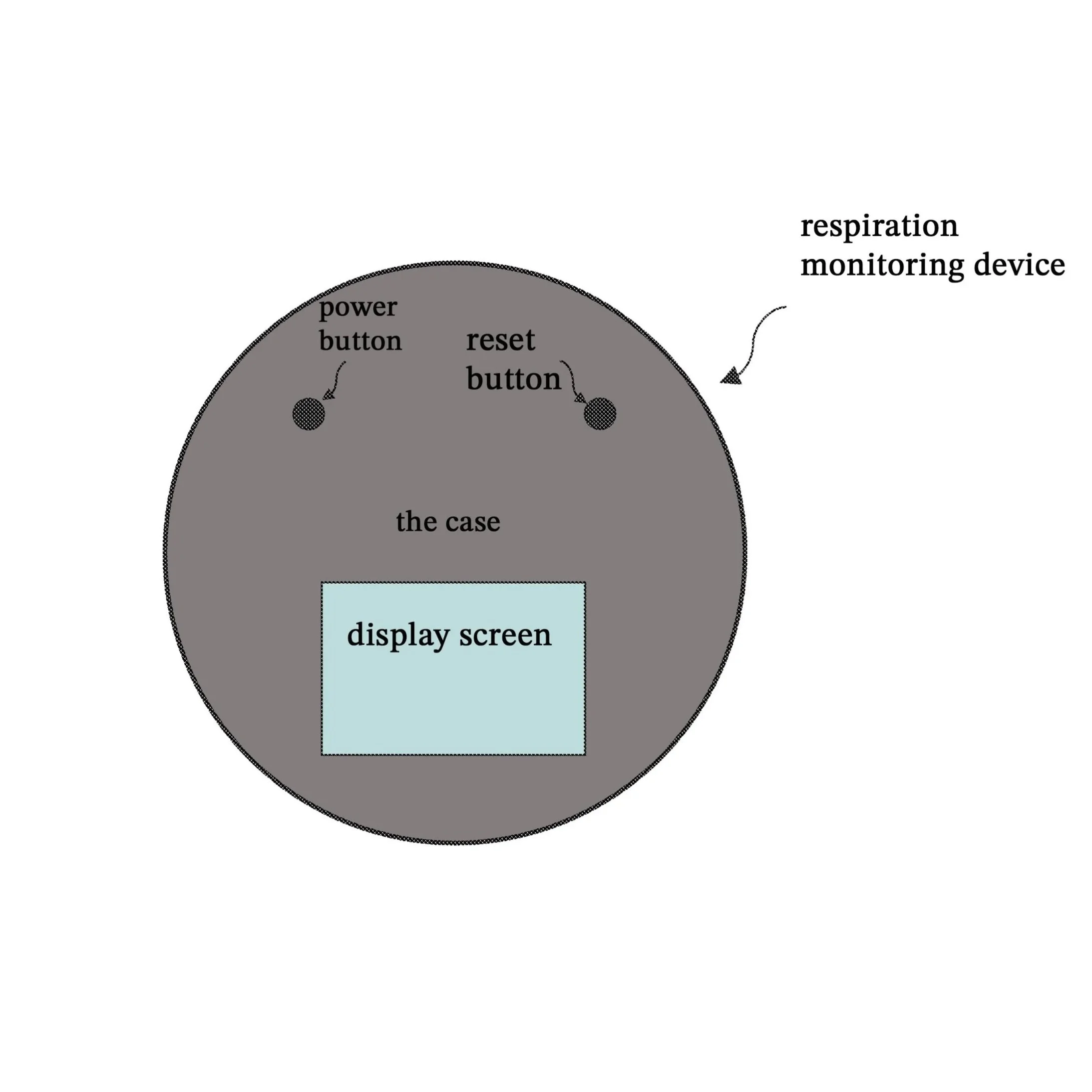
Respiration Monitoring Device
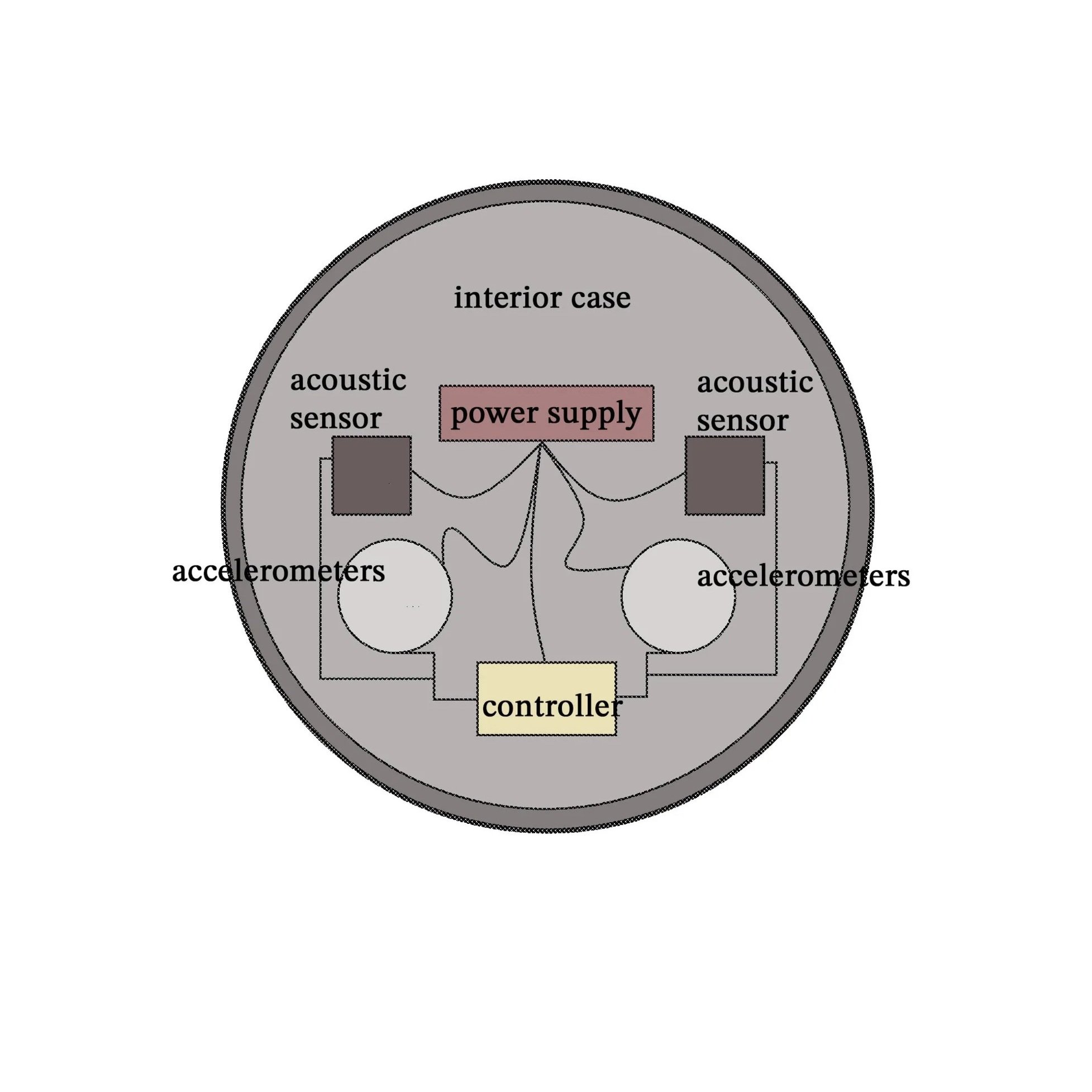
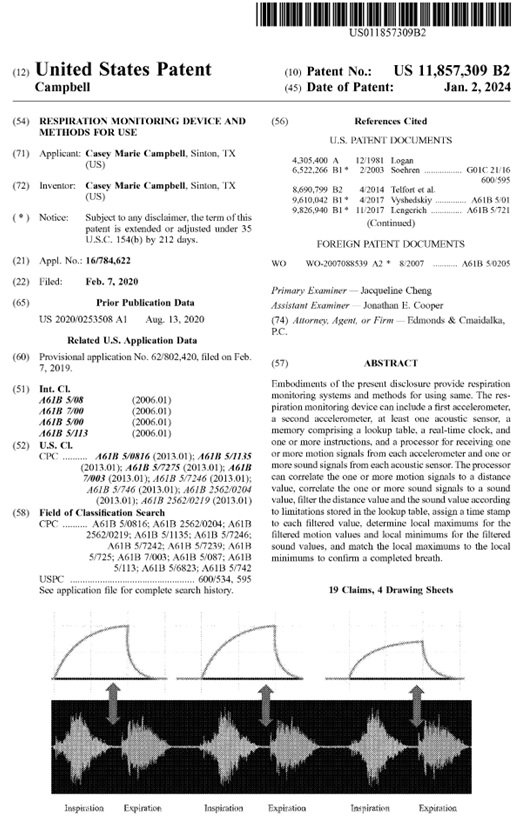
The Respiration Monitoring Device is an innovative system designed to provide precise, real-time monitoring of an individual’s breathing patterns. The device integrates advanced sensor technologies, including dual accelerometers and one or more acoustic sensors, to detect both physical movement and sound associated with respiration. Supported by a built-in memory containing a lookup table, a real-time clock, and programmed instructions, the system processor intelligently analyzes incoming motion and sound signals to extract accurate respiratory data.
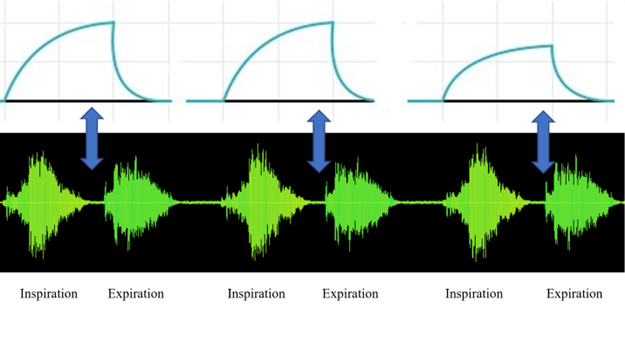
Through signal correlation, filtering, and time stamping, the processor identifies key respiratory events by matching local maximums of motion data with corresponding local minimums of acoustic data, thereby confirming each completed breath. This dual-sensor verification process enhances accuracy and reduces false readings, ensuring dependable results across various environments and activity levels.
The Respiration Monitoring Device represents a significant advancement in health monitoring technology. By combining motion and sound analysis into a compact, automated system, this device sets a new standard for reliability, precision, and user convenience in continuous respiration tracking.
PRODUCT FEATURES & BENEFITS
Special Features
Real-time respiration monitoring
Automatic breath detection and confirmation
Time-stamped data recording
Rechargeable battery system
Customizable breathing alerts
The Respiration Monitoring Device is a compact, non-invasive tool designed primarily for vital sign acquisition in hospital and clinical settings. Intended for short-term use, typically less than one minute, the device is placed on a patient’s chest to quickly and accurately obtain a respiratory count during routine vital sign collection.
PRODUCT DETAILS
By correlating both motion and sound data, it delivers a faster and more reliable way to measure respiration compared to manual counting, helping clinicians save time while maintaining precision. This makes it especially useful for monitoring patients with respiratory conditions, post-operative recovery needs, chronic illnesses requiring periodic assessment, or early identification of deteriorating patients for prompt medical intervention.
Engineered for ease of use, hygiene, and clinical accuracy, the Respiration Monitoring Device is ideal for doctors’ offices, hospitals, and other point-of-care environments. Its temporary placement ensures optimal performance for short, focused respiratory assessments.
Materials needed to produce the Respiration Monitoring Device:
▪ Medical-grade silicone
▪ Polycarbonate (PC)
▪ Acrylonitrile Butadiene Styrene (ABS)
▪ Thermoplastic polyurethane (TPU)
▪ Aluminum alloy
▪ Stainless steel (316L)
▪ Copper
▪ Gold-plated connectors
▪ Printed circuit board (PCB)
▪ Lithium-ion or lithium-polymer battery
▪ Polyester or PTFE acoustic membrane
▪ Medical-grade adhesive pads
▪ Silicone or rubber sealing gasket
▪ Optical-grade acrylic window
The Respiration Monitoring Device can elevate your product portfolio to new heights. We are seeking a long-term arrangement with a company to manufacture, market, and/or distribute this new technology based on the acquisition of the intellectual property rights . Contact us below
Contact Us
Our VP of Business Development, Amy Schleicher, is ready to answer any questions you may have.
Phone: 913-201-8025
E-mail: Amy@bankonip.com
Patent Information
Patent
Inventor Highlight
Casey Campbell holds United States Utility Patent No. 11,857,309, filed on Feb. 07, 2020, and issued on Jan. 02, 2024. The patent for the Respiration Monitoring Device expires in Sept. 2040, commensurate with the filing date, and has 19 claims that protect the exclusive design and/or function of the Respiration Monitoring Device.
Social Media
Follow us in real time! Stay connected.
“There’s a way to do it better—find it”